M1Q3 Classification of MatterAs a simple example of VSEPR theory, let us predict the structure of a gaseous BeF 2 molecule The Lewis structure of BeF 2 shows only two electron pairs aroundSummary of VSEPR Film and Lecture Steric Number = Number of Bonded atoms plus Lone Pairs S# bond angle Hybrid orbital type 4 109½ o sp 3 hybrid orbitals (4
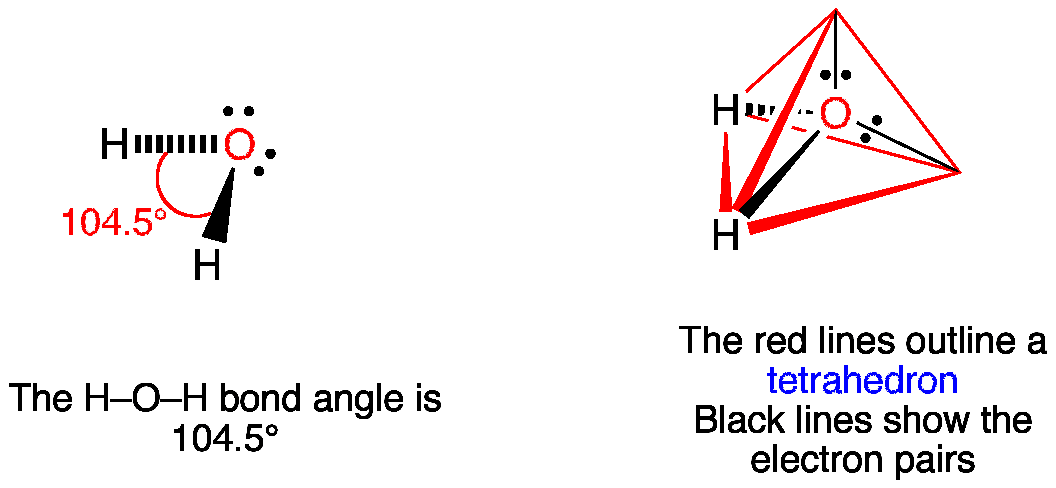
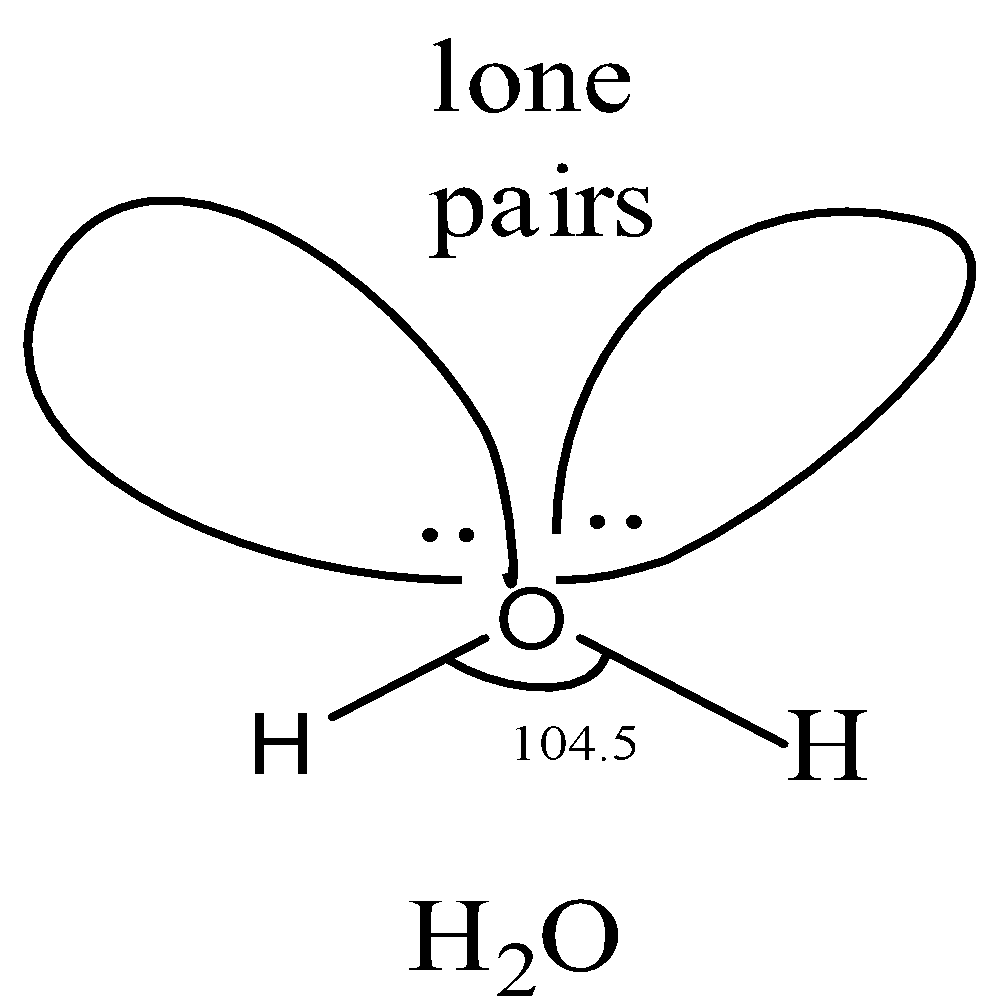
Vsepr H2o Water
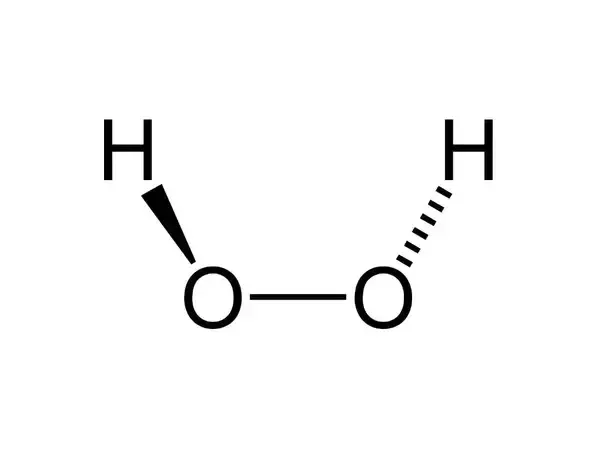
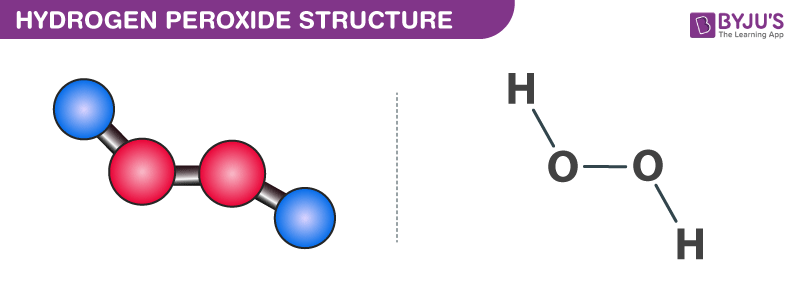
Vsepr hydrogen peroxide structure
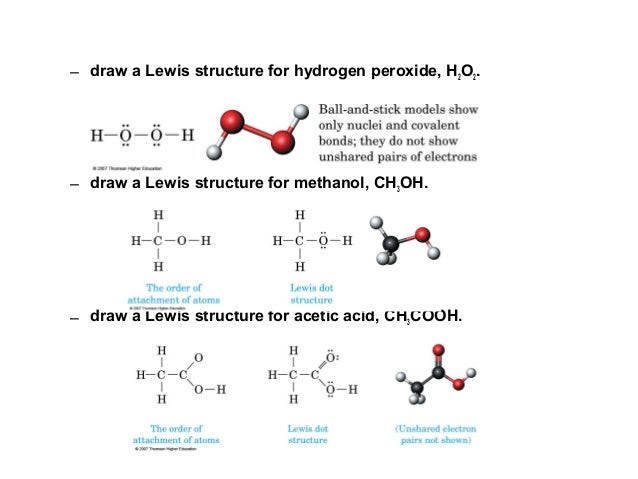
Vsepr hydrogen peroxide structure-N 2 H 4 Hydrazine N has 5 valence electrons plus 1 for each NH or NN single bond Total = 8 electrons, four pairs Structure each nitrogen is trigonal pyramidWrite a Lewis structure for an atom of each element Carbon Write the Lewis structure Show all valence electrons H2O2 Hydrogen peroxide Write the Lewis


Steric Number And Bond Angles
The least electronegative atom is placed in the center of a Lewis structure because it will be more likely to share its electrons with more than one atom a singleDIn terms of and bonds, describe the two oxygenoxygen bonds in the Lewis structure 1 The two oxygenoxygen bonds in ozone are in fact of equal length Deduce whyStructure of Hydrogen Peroxide The structure of hydrogen peroxide is nonplanar H 2 O 2 has an open book structure with O – O spins The dihedral angle is
VSEPR Theory (Molecular Shapes) A = the central atom, X = an atom bonded to A, E = a lone pair on A Note There are lone pairs on X or other atoms, but we don't careHydrogen Peroxide Hydrogen peroxide is a polar molecule having the molecular formula H2O2 H 2 O 2 It is a strong oxidizing agent that is used to oxidize phenolsFree Question Bank for JEE Main & Advanced Chemistry Chemical Bonding and Molecular Structure / रासायनिक आबंधन एवं आणविक संरचना VSEPR theory Hydrogen
So each hydrogen atom now sees 2 electrons when it is covalently bonded to another hydrogen atom Pure hydrogen exists as H 2 molecules The same is true for allHence, although quite a few students incorrectly tried to reflect the delocalization of ozone in their Lewis structures in part (a), their answers to the later partsThe N2F2 molecule has the two nitrogens as the central atoms State the


Making Molecules Lewis Structures And Molecular Geometries Annenberg Learner



Ammonia Chemical Polarity Molecule Ammonium Chemistry B Purple Sphere Chemistry Png Klipartz
Sep 16, 04 · Methylamine can be converted by semicarbazidesensitive amine oxidase (SSAO) to formaldehyde and hydrogen peroxide, which have been proven to be toxic towardsHydrogen peroxide is a colorless liquid at room temperature with a bitter taste Small amounts of gaseous hydrogen peroxide occur naturally in the air HydrogenMay 04, · Vsepr H2o Water Hydrogen Peroxide Structure Properties Uses With Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Is H2o2 Polar


Is H2o2 Polar Or Non Polar Quora



Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book
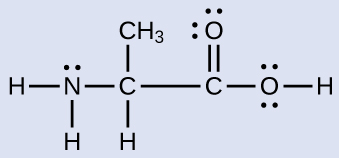
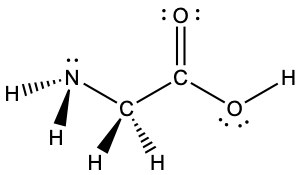
The oxygen atoms are sp3 hybridized The VSEPR theory clearly states that sp3 hybridized atoms will adopt a tetrahedral arrangement of electron pairs around it FirstLewis electron dot structure (draw resonance structures if they exist) 12 NH4 13 SO4 2– 14 N2 15 CHCl3 Chloroform 16 C2H6 Ethane 17 CO2 18 CH3OH Methanol 19 H2O2M1Q2 States of Matter;


H2o2 Lewis Structure Hydrogen Peroxide Molecular Geometry Polarity



Vishal Goyal Topblogtenz Profile Pinterest
ਵੈੱਬ ਸਾਇਟ ਤੇ ਉਪਲੱਬਧ ਪਾਠਪੁਸਤਕਾਂ ਕੇਵਲ ਅਧਿਆਪਕਾਂ, ਵਿਦਿਆਰਥੀਆਂ ਅਤੇ ਮਾਪਿਆਂ ਦੀ ਵਰਤੋਂ ਲਈ ਹਨ ਇਨ੍ਹਾਂ ਪਾਠਪੁਸਤਕਾਂ ਦੀ ਦੁਰਵਰਤੋਂ (ਜਿਵੇਂ ਪ੍ਰਿੰਟ ਕੱਢ ਕੇ ਵੇਚਣਾ ਆਦਿ) ਕਰਨਾView Chapter 3 part 2pdf from CHEM 1040 at Brooklyn College, CUNY Bettelheim / Brown / Campbell / Farrell / Torres Introduction to General, Organic, and BiochemistryCan anyone show me the VSEPR and Valence Bond sketch of H2o2 Hydrogen peroxide?


Hydrogen Peroxide Molecule Of The Month September 06 Html Version


Molecular Structure And Polarity Chemistry 2e
Lecture 441 – KösselLewis Approach to Chemical Bonding (Octet Rule, Lewis Dot Structures and Formal Charge) Lecture 442 – Resonance Lecture 451 – Valence ShellMar 21, · The Shape Of The Hydrogen Peroxide Molecule In The Gas Phase Dihedral Angle Of Gaseous And Crystalline Hooh Chemistry Is H2o2 Polar Or Non Polar Quora HydrogenThis model structure shows the 1o trigonal bond angles formed by a central sphere & 3 smaller terminal spheres The entire model is approximately 150mm (6") tall



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Stock Photo Picture And Royalty Free Image Image


7 6 Molecular Structure And Polarity Chemistry
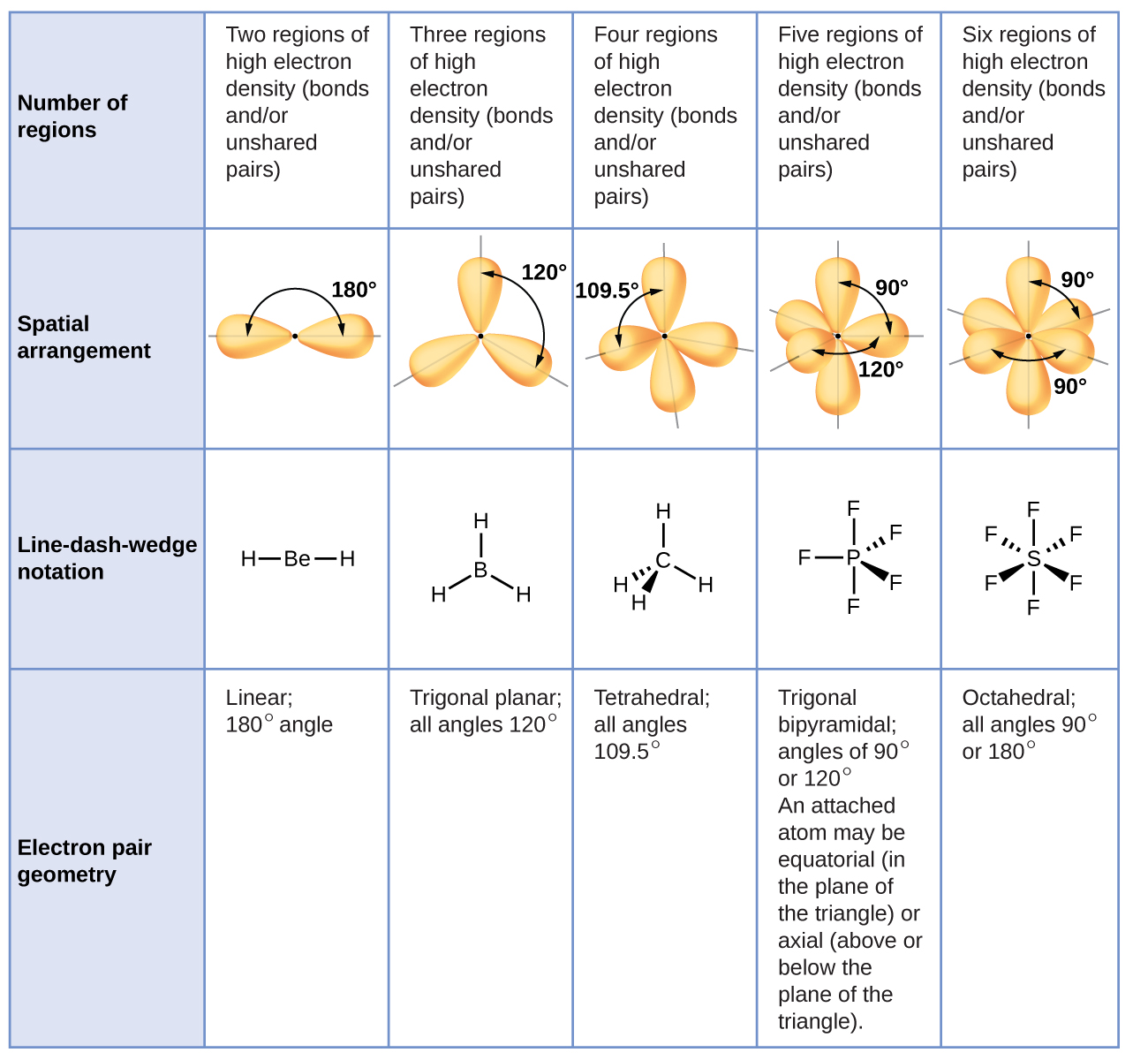
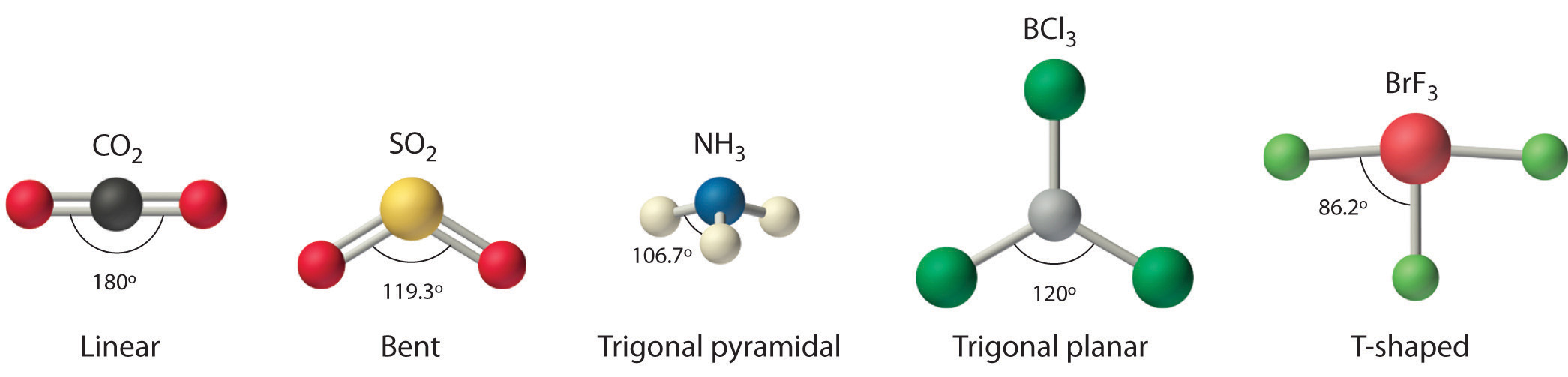
Water has 4 regions of electron density around the central oxygen atom (2 bonds and 2 lone pairs) These are arranged in a tetrahedral shape The resulting molecularA peroxide is any compound which has two oxygen atoms bonded together The OO group is the peroxide group of the compound And Hydrogen Peroxide is the simplestThe valenceshell electronpair repulsion (VSEPR) model allows us to predict which of the possible structures is actually observed in most cases It is based on


Hydrogen Peroxide Structure Uses And Properties Of Hydrogen Peroxide



Vsepr H2o Water
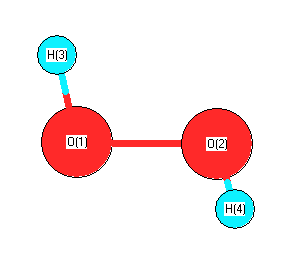
Feb 25, 11 · Hydrogen peroxide has two equivalent central atoms with two unshared pairs and two single bonds This creates an angled bond, creating the bent shape of the moleculeNov 30, 12 · C 2 H 4 is the symbol for the simple organic compound, ethylene It is the simplest alkene (hydrocarbon with carboncarbon double bonds)) This illustrationMay 25, 10 · H2O2 has a special shape, it has an open book structure VSEPR is all about valence, as the abbreviation suggests like you said, the idea is that lone pair/lone pair >



Makethebrainhappy Is H2o2 Polar Or Nonpolar


H2o2 Lewis Structure Youtube
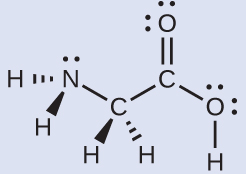
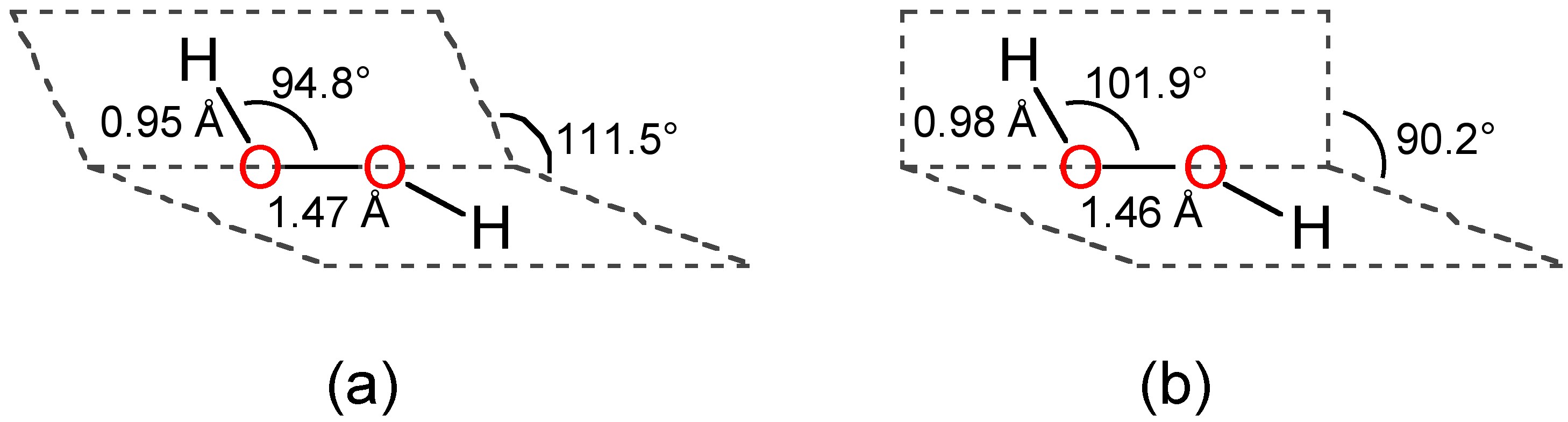
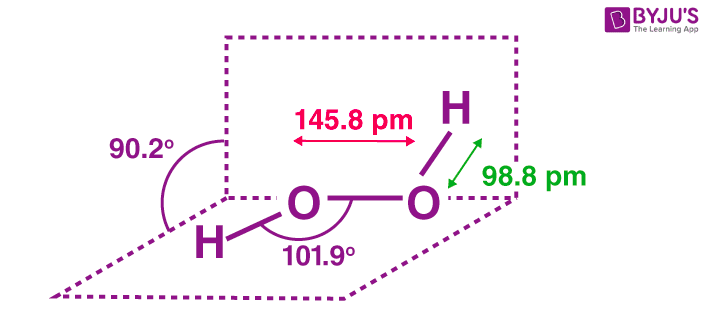
It has a skewed structure with a dihedral angle of 1115° (gas phase), which minimises repulsion between the lone pairs and the OH bond pairs The dihedral angle isHydrogen peroxide ({eq}H_2O_2 {/eq}) Lewis Structure Then, learn how to predict the shape of a molecule by applying the VSEPR theory to the Lewis dotUse the Orbit 681W point group molecular model kit to build hydrogen peroxide and show C2 point group symmetry The hydrogen peroxide molecule can undergo an


H2s Lewis Structure Molecular Geometry Hybridization And Polarity


Hydrogen Peroxide Eagle Marketing
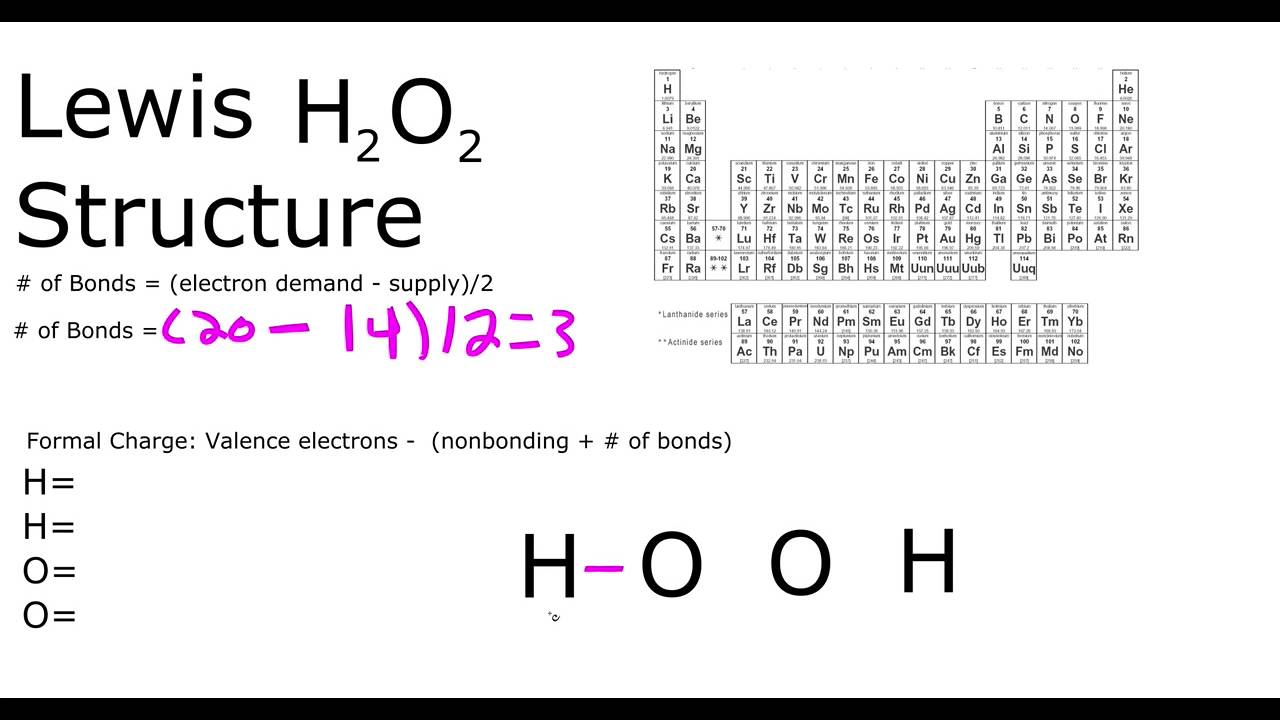
Another limitation of VSEPR theory is that it predicts that halides of group 2 elements will have a linear structure, whereas their actual structure is a bent oneA stepbystep explanation of how to draw the HOOH Lewis Dot Structure For the HOOH Lewis structure, calculate the total number of valence electrons for theThe VSEPR models of molecules can be found systematically by using the number of electron pairs to determine the shape of the molecules To predict the shape of the


Vsepr



Vsepr Chart Valence Shell Electron Pair Repulsion Theory Sigma Aldrich
Structure, properties, spectra, suppliers and links for Hydrogen peroxide, , , , HOOHModule 1 Introduction to Chemistry Concepts M1Q1 Measurements, Units, Conversions, Density;Jul 27, 19 · The VSEPR theory is able to predict geometry of a large number of molecules, especially the compounds of pblock elements accurately It is also quite successful in


Molecular Structure And Polarity Chemistry 2e


Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book
Solution The Lewis structure for OF 2 is F δδ O F There are no formal charges on the atoms in OF 2, but F is more electronegative and will "pull" the electronsThe monomeric form adopts a bent structure very similar to that of sulfur dioxide with a bond length of 161 pm The dimeric form has been isolated in a low temperatureSep 01, 16 · $\begingroup$ The conformation of hydrogen peroxide (H2O2) is dominated by the lone pairs rather than the hydrogen atoms Instead of the expected anti conformation



Determine The Geometry About Each Interior Atom In Each Molecule And Sketch The Molecule Skeletal Structure Is Indicated In Parentheses A Ch 3oh H 3coh B Ch 3och 3 H 3coch 3 C H 2o 2 Ho Study Com


Hydrogen Peroxide Molecule Of The Month September 06 Html Version
Phosphorus trichloride, PCl hydrogen peroxide, H2O AE Lewis structure AE Lewis structure NE NE VSEPR sketch VB sketch VSEPR sketch VB sketch geometryThe structure of dioxygen difluoride resembles that of hydrogen peroxide, H 2O 2, in its large dihedral angle, which approaches 90° and C 2 symmetry This geometryLewis Structures for Covalent Molecules 2) Arrange the atoms and link with single bonds the more bond an atom can form, the more center it will be placed;


Predicting Molecular Shapes Vsepr Model M9q1 Uw Madison Chemistry 103 104 Resource Book


Cccbdb Listing Of Experimental Data Page 2
According to the Hydrogen peroxide lewis structure, it contains a total of 4 lone pairs and each oxygen (central atom) has 2 lone pairs Or you can determine lone pair


Electron Pair Geometry


Lewis Structure Vsepr Theory Water Structural Formula Chemical Bond Png 770x404px Lewis Structure Area Black Black


Molecular Structure And Polarity Chemistry
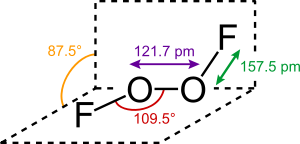


Dioxygen Difluoride Wikipedia



Vsepr Theory
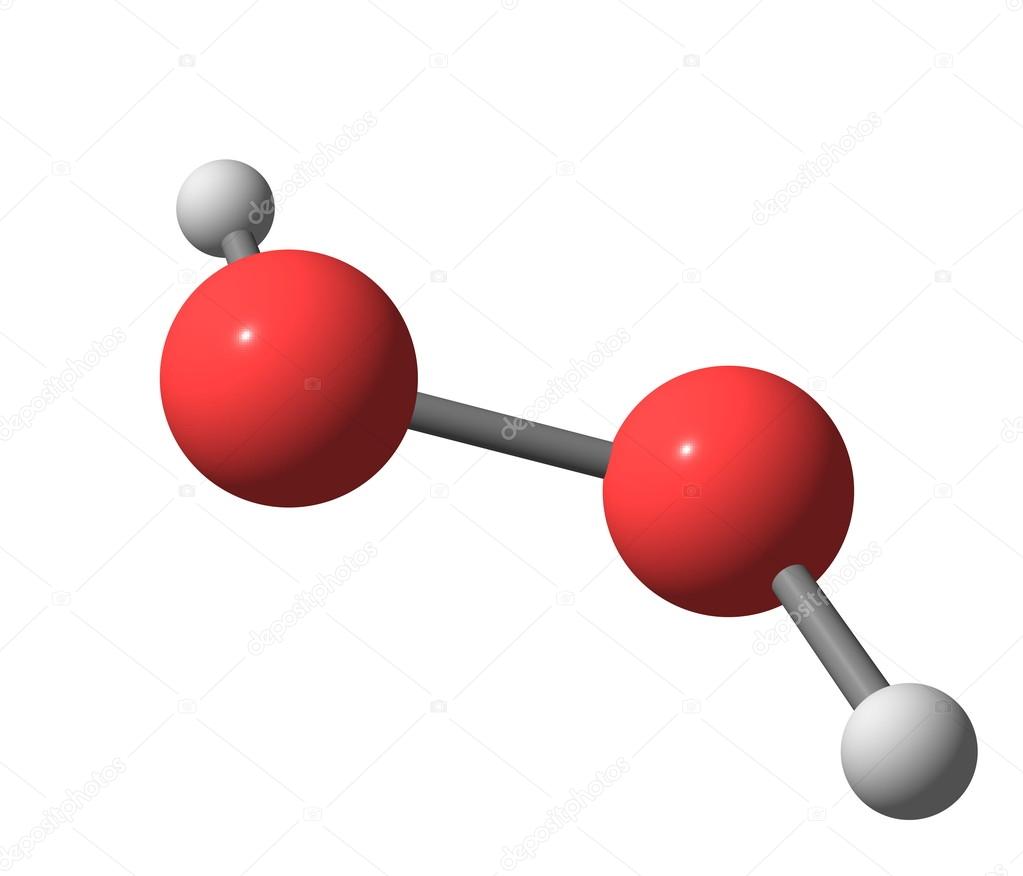


Hydrogen Peroxide H2o2 Molecular Structure Isolated On White Stock Photo Image By C Olla Davies


7 E Chemical Bonding And Molecular Geometry Exercises Chemistry Libretexts
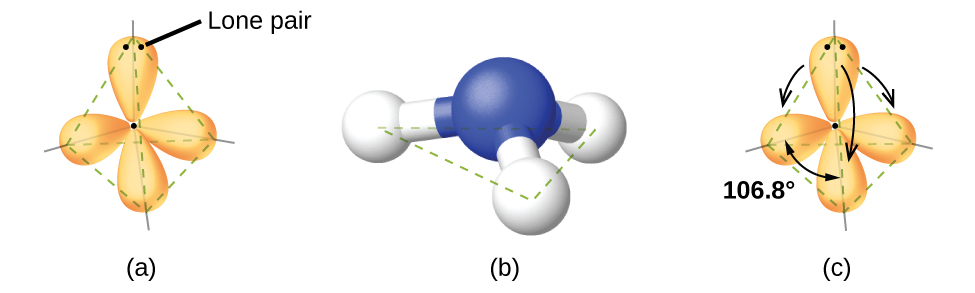


7 6 Molecular Structure And Polarity Chemistry
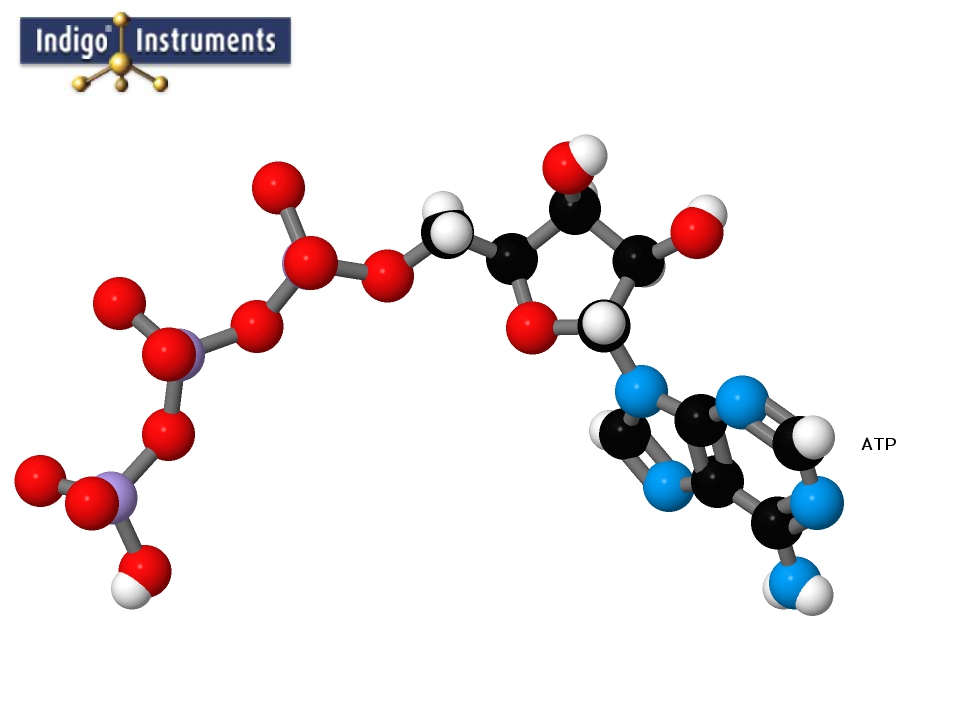


Atp Nucleotide Molecule Structure Model Built With Molymod Atoms Bonds From Indigo Instruments



Oxygen Ozone Molecule Hydrogen Peroxide Atom Others Atom O 2 O 3 Png Pngwing



Solved Viosios Leo Hydronium Ion H O Ae 9 Lewis Structur Chegg Com



Valence Shell Electron Pair Repulsion Vsepr Theory Chemistry Of The Main Group Elements Openstax Cnx



Solved Phosphorus Trichloride Pcl Hydrogen Peroxide H2o Chegg Com


Illustrated Glossary Of Organic Chemistry Hydrogen Peroxide H2o2 Hooh



Why H2o2 S Molecular Geometry Is What It Is Chemistry Stack Exchange



H2o2 Molecular Geometry Shape And Bond Angles See Descp For Precise Angles Youtube
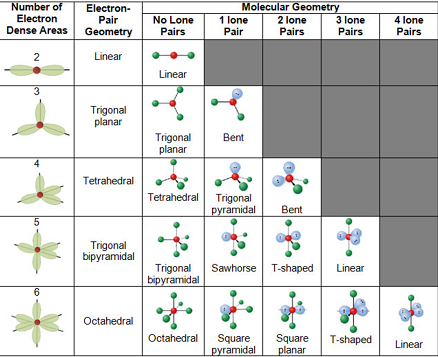


Vsepr Theory Postulates Limitations Predicting Shapes



Bent Molecular Geometry Png Images Pngwing


Steric Number And Bond Angles
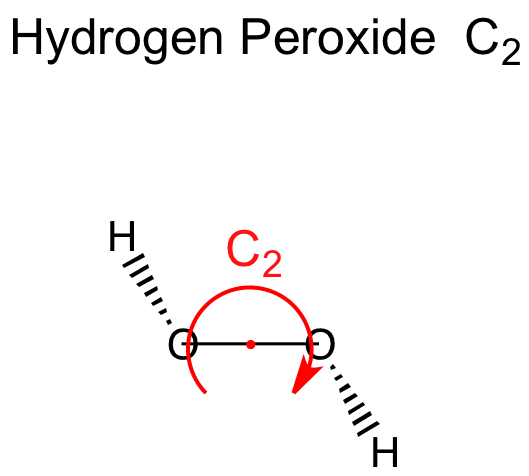


Hydrogen Peroxide C2



Hydrogen Peroxide Structure Properties Uses With Questions Videos



What Is The Lewis Structure For H2o2 Study Com



Molecular Shapes And Polarity Introductory Chemistry 1st Canadian Edition



H2o2 Vsepr



What Is The Oxidation Number Of Oxygen In O2f2 Quora
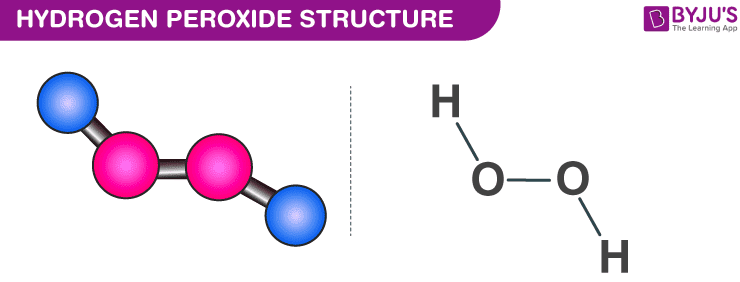


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses
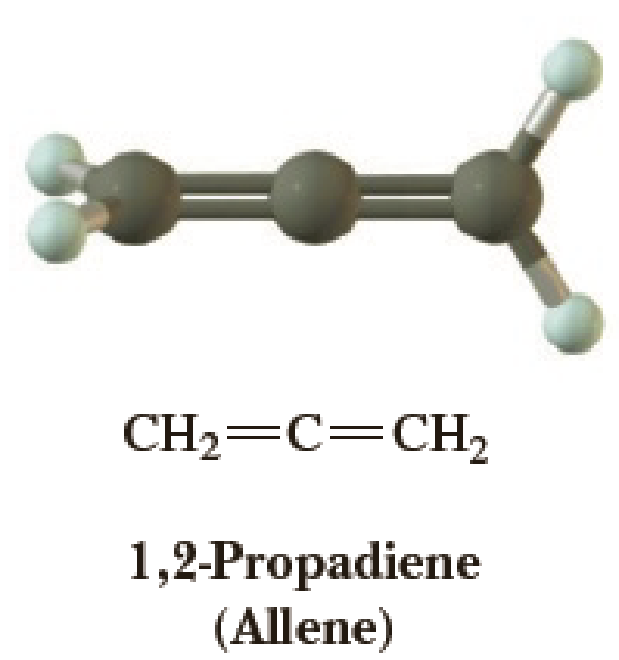


The Structure Of 1 2 Propadiene Allene Is Shown To The Right A Predict All Approximate Bond Angles In This Molecule B State The Orbital Hybridization Of Each Carbon C Explain The Three Dimensional Geometry



Hydrogen Peroxide H2o2 Lewis Structure Novocom Top


H2o2 Vsepr



Vsepr H2o2 Structure Novocom Top



Vishal Goyal Topblogtenz Profile Pinterest


7 6 Molecular Structure And Polarity Chemistry



Dioxygen Difluoride Wikiwand



Why H2o2 S Molecular Geometry Is What It Is Chemistry Stack Exchange
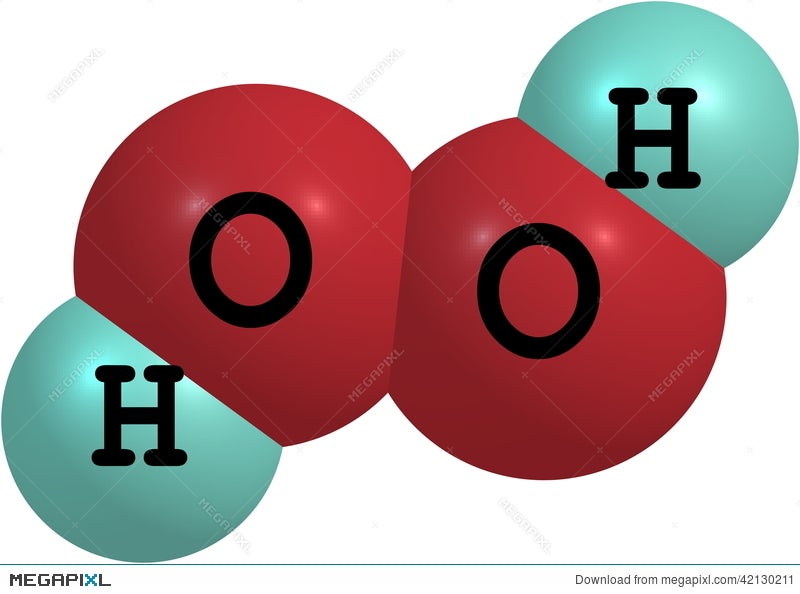


Hydrogen Peroxide H2o2 Molecular Structure Isolated On White Illustration Megapixl



Vsepr Theory Molecule Trigonal Planar Molecular Geometry Linear Molecular Geometry Trigonal Sphere Chemistry Atom Png Pngwing



Is H2o2 Polar Or Nonpolar Techiescientist


9 4 Hydrogen Peroxide Chemistry Libretexts


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses



Chemical Bond Adv



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Royalty Free Cliparts Vectors And Stock Illustration Image



Hydrogen Peroxide



H2o2 Lewis Structure And Vsepr Model Novocom Top



H2o2 Geometric Structure Hydrogen Peroxide Formula



Is H2o2 Polar Or Nonpolar Youtube


Why Does The Extra Oxygen Atom In Hydrogen Peroxide H2o2 Make It An Antiseptic While Water H2o Is Not Antiseptic Quora



Mgcl2 Linear Molecule Geometry Vsepr Theory Model



Molecular Shape Britannica


Sodium Peroxide Wikipedia



Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Youtube



Sulfoxide Wikipedia



H2 Lewis Structure Novocom Top



Solved 09 Molecular Geometry And Bonding 219 352 4 17 S Chegg Com


Phosphoric Acid H3po4 Pubchem


Chemical Makeup Of Hydrogen Peroxide Saubhaya Makeup


Chapter 3


Molecular Geometry And Covalent Bonding Models



Redox Reactions Kaiserscience


Bond Angles And The Shapes Of Molecules


Rabbit Ears Hybrids Vsepr Sterics And Other Orbital Anachronisms Chemistry Education Research And Practice Rsc Publishing



Linear Molecular Geometry Vsepr Theory Molecule Chemistry Molecular Geometry Png Pngegg


Compare The Structures Of H2o And H2o2 Class 11 Chemistry Cbse


Bond Angles And The Shapes Of Molecules
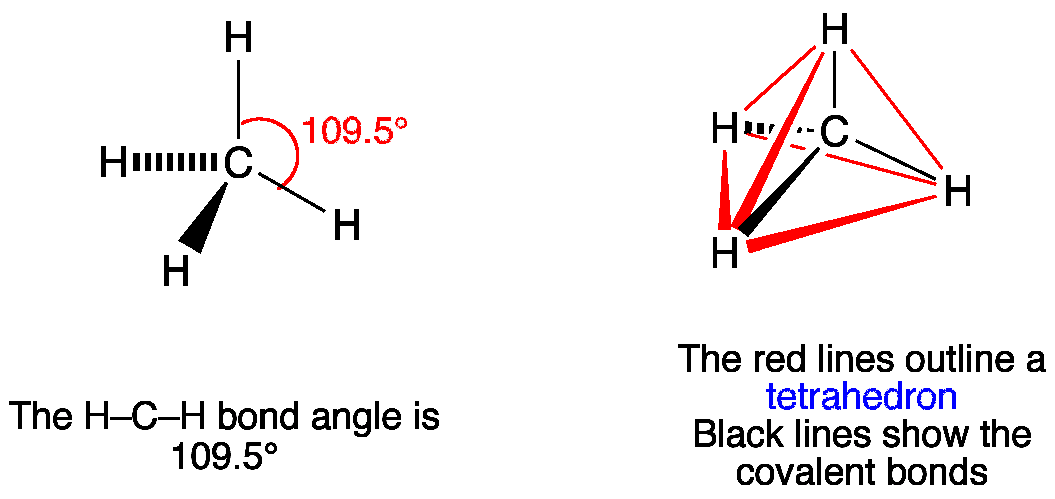


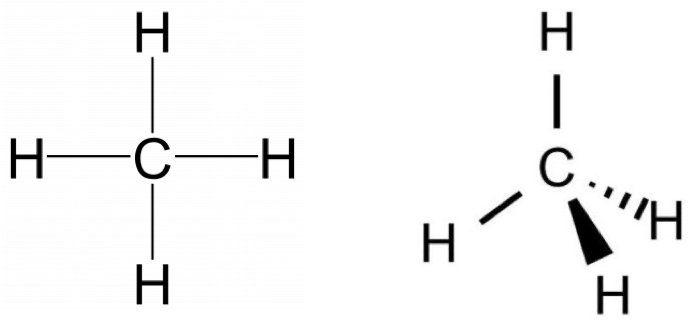
Vsepr Ch4 Methane
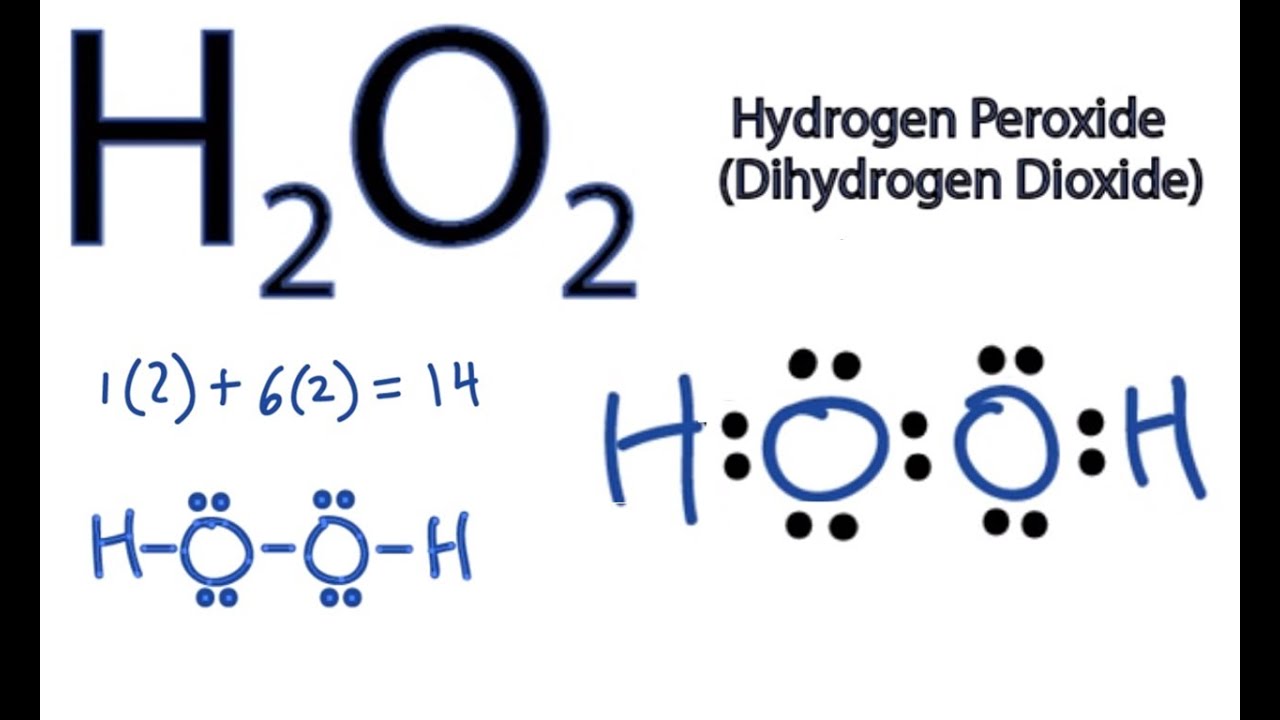


Is H2o2 Polar Or Nonpolar Youtube


Molecular Structure And Polarity Chemistry


Hydrogen Peroxide H2o2 Chemspider



Hydrogen Peroxide H2o2 Structural Formula 3d Stock Illustration



Sulfur Dioxide Lewis Structure Molecule Molecular Geometry Resonance Silicon Dioxide Structure Angle Text Chemistry Png Pngwing
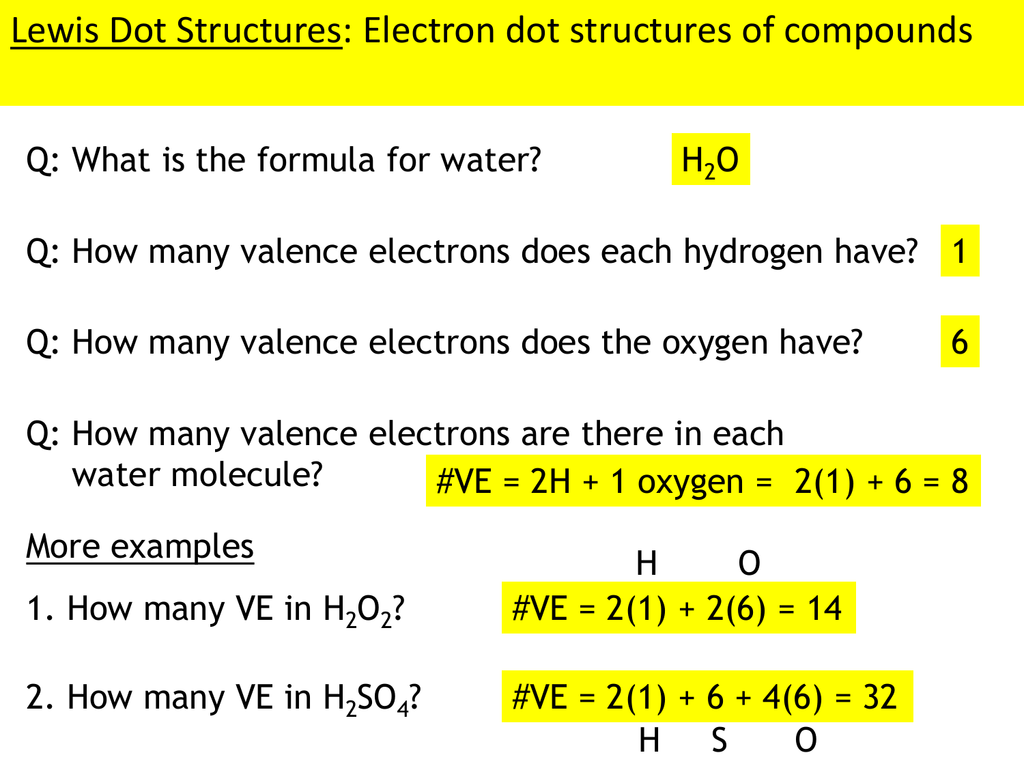


Lewis Dot Structures



Valence Shell Electron Pair Repulsion Vsepr Theory Chemistry Of The Main Group Elements Openstax Cnx
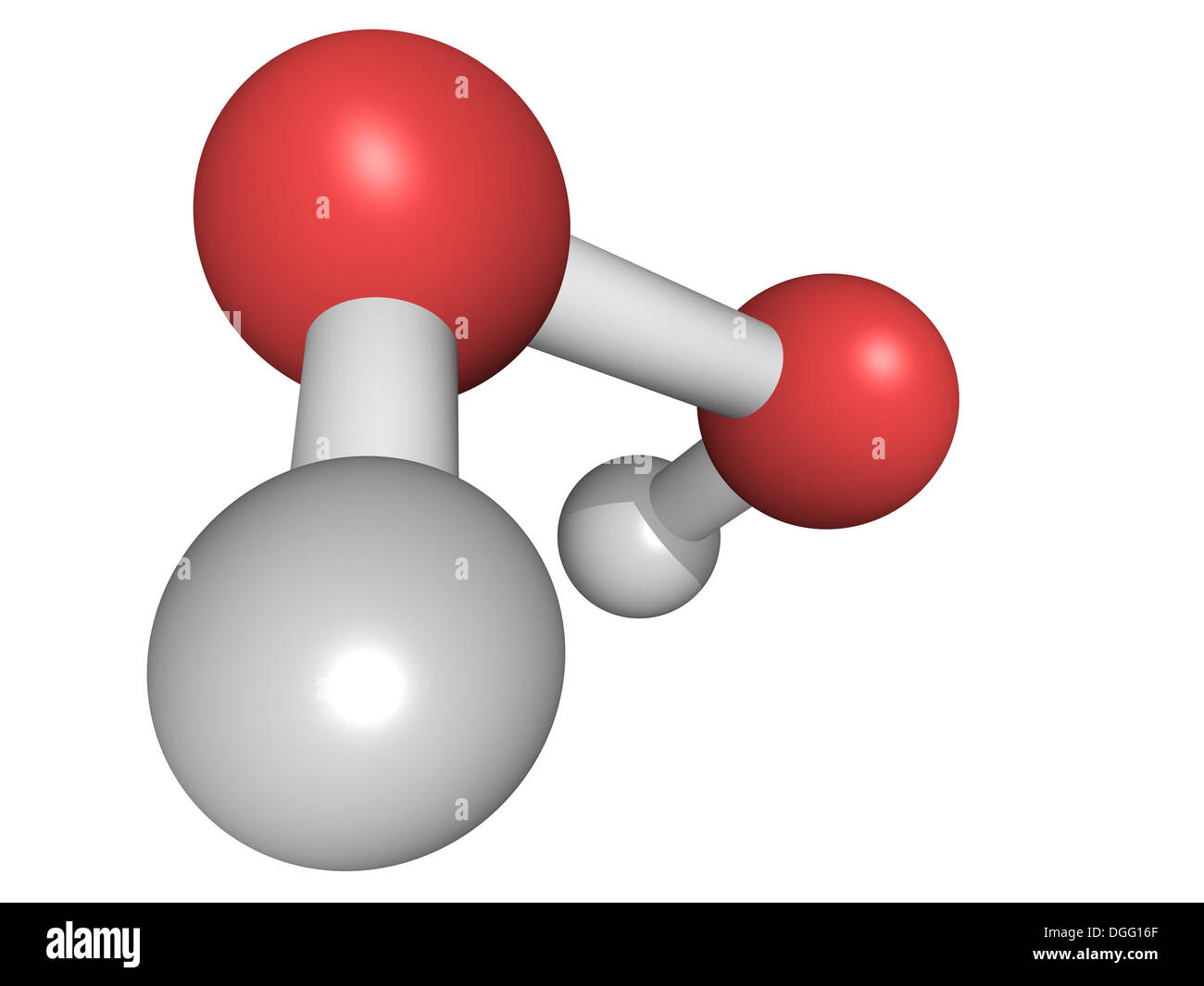


Chemical Structure Of A Hydrogen Peroxide H2o2 Molecule Hooh Is A Reactive Oxygen Species Ros Used In Bleach And Cleaning Stock Photo Alamy
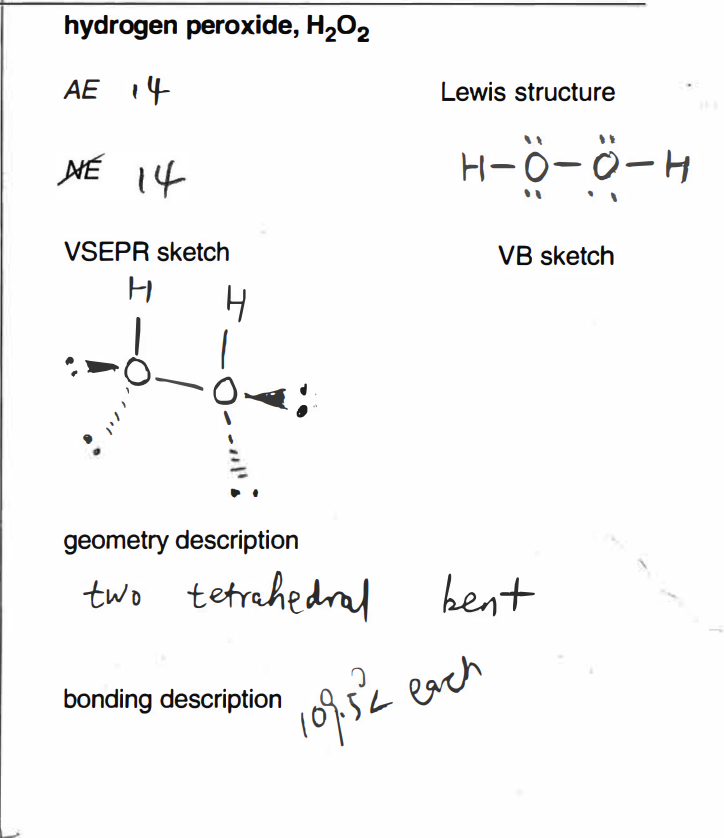


Solved I Added An Example Provided By My Educator I Am C Chegg Com



0 件のコメント:
コメントを投稿